Is Magnesium A Pure Substance Or Mixture The Mster Minerl
Any material that is not a mixture, is called a pure substance. See examples of other pure substances and mixtures, and how to classify them as homogeneous or. A shiny magnesium ribbon is burned in air, to form a.
Magnesium — Science Learning Hub
A pure substance has a definite and constant composition — like salt or sugar. By definition, a pure substance or a homogeneous mixture consists of a single phase. There are 118 elements found in the periodic table.
Magnesium is classified as a pure substance because it is a chemical element with the symbol mg and atomic number 12.
If a substance can be separated into its elements, it is a compound. Is magnesium ribbon a heterogeneous mixture?. If the material is a pure substance, further classify it as either an element or compound in the right column. Magnesium is a pure substance, specifically an element, according to chemteam.
In the center column, state whether the material is a pure substance or a mixture. Yes, magnesium oxide is a pure substance or mixture. When oil and water are combined,. A substance made of atoms that all contain the same number of protons and cannot be split into anything simpler.

Magnesium Description, Properties, & Compounds Britannica
While pure substances have clearly defined physical and chemical properties, mixtures have different properties, depending on the proportions of the pure substances in.
It is much more difficult to break down pure substances into their parts, and. Magnesium glycinate is an oral magnesium supplement that helps restore nutrient levels in the body. Classify each of the following as a pure substance or a mixture. It’s a homo mixture because it’s made up of more than one substance.
In everyday language, we use the word pure to describe when something is natural or clean and to which nothing else has been added. If it is pure, the substance is either an element or a compound. It is an inorganic compound that occurs in nature as the mineral periclase. A heterogeneous mixture consists of two or more phases.

Magnesium — Science Learning Hub
In contrast, mixtures consist of two or more different substances that retain their individual properties and can be separated by physical means.
Pure substances include elements and compounds. It is a pure substance and cannot be classified as a compound, heterogeneous mixture, or homogeneous mixture. Magnesium is an element on the periodic table. All matter can be classified as either a pure substance or a mixture.
If a mixture, indicate whether it. Decide whether a substance is chemically pure. Because of its high bioavailability. Is magnesium metal a homogeneous mixture?
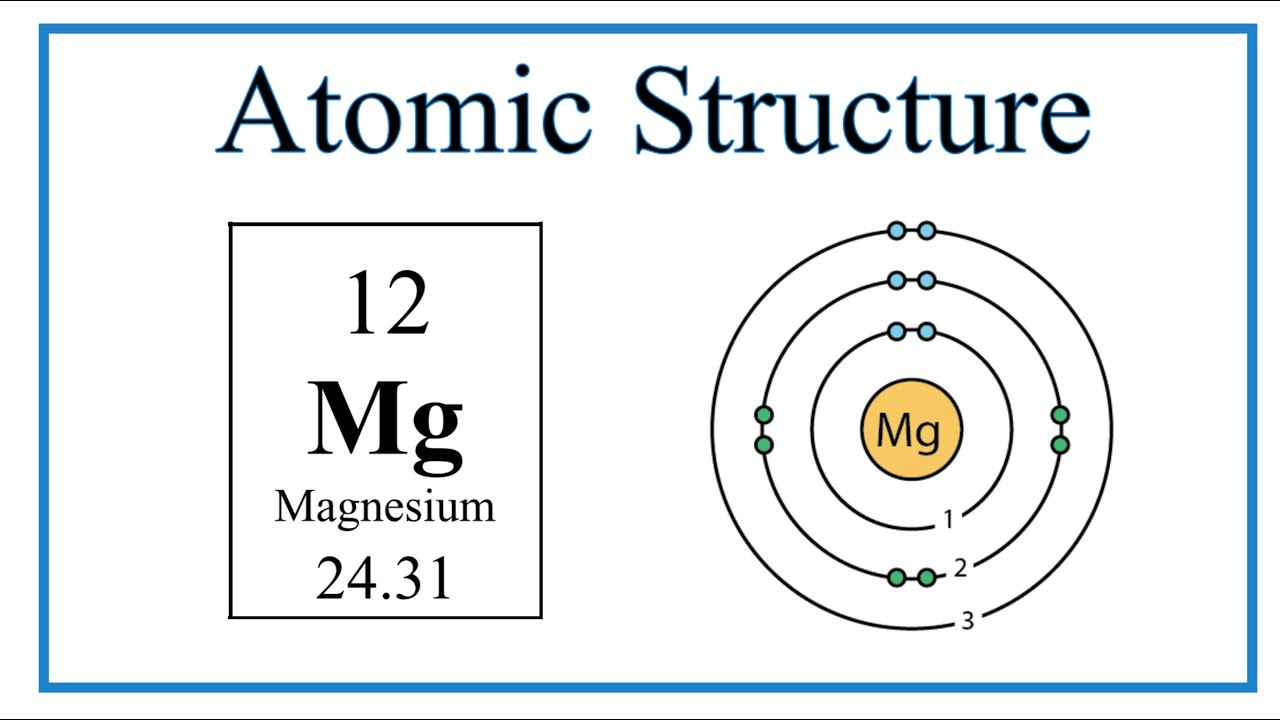
Diagram Of The Atomic Structure Of Magnesium Chemical Elemen