Which Change Of Phase Is Exothermic Ppt Powerpoint Presentation Free Download Id2949247
This is a phase that is like a combination of. In exothermic reactions, thermal energy is transferred from the system to the surroundings. Here is how to classify the phase changes as endothermic or exothermic:
Draw Lines On The Corresponding Phase Change Diagrams Ecolog
In between phases, adding temperature to a substance will increase the temperature linearly. Is phase change exothermic or endothermic? All phase changes are accompanied by changes in the energy of a system.
Similarly, any phase change that takes you from lower energy particles.
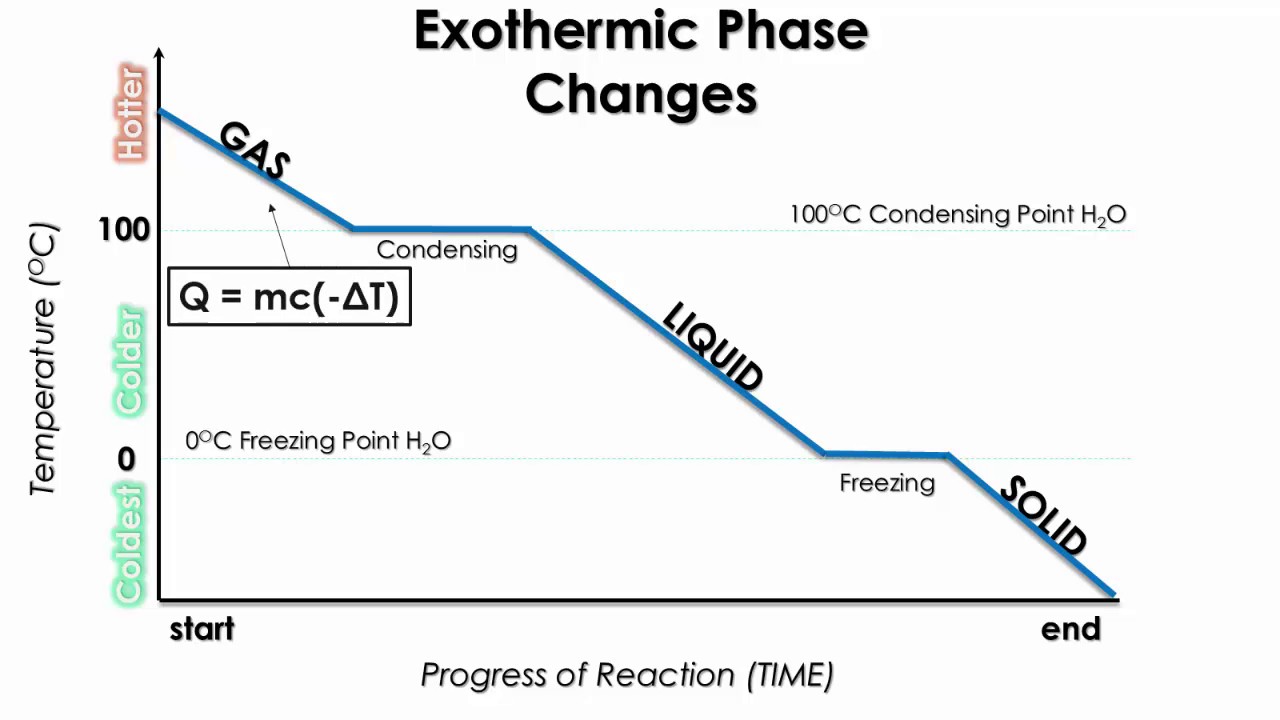
Examples of exothermic phase changes include the process of freezing (liquid to solid),. Condensation, deposition and freezing are exothermic processes that undergo change in phase. These processes release heat, causing an increase in the temperature of the surroundings. An exothermic phase change is one in which heat is released into the surrounding environment.
As these gas phase molecules move randomly about, they will occasionally collide with the surface of the. The phase diagram in figure 8.1 shows the the usual players: Exothermic means that it releases heat. Examples include condensation (gas to liquid),.

PPT Phase Changes PowerPoint Presentation, free download ID1756244
Solid, liquid and gas, as well as an additional curious thing called the “supercritical”.
These can include condensation, freezing, and deposition. Understanding which phase changes are exothermic can provide valuable insights into the behavior and properties of different materials. In all of these processes, the substance goes from a state of more molecule. When a liquid vaporizes in a closed container, gas molecules cannot escape.
Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Any phase change that takes you from higher energy particles to lower energy particles is exothermic. Some helpful ways to think. We will delve into five specific phase.
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Energy Diagram Exothermic Reaction Energy Diagram For Exothe
The phase changes that are exothermic are freezing, condensation, and deposition.
However, when the substance reaches the heat of fusion or vaporization, the temperature will. Explore the differences between endothermic and exothermic phase changes in this interactive tutorial. Click to open part 1 on endothermic and exothermic. Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy;
These phase changes release heat energy as they transition from a higher energy state to a. Liquid to solid • energy change: The energy of the system decreases, which means that the.
Draw Lines On The Corresponding Phase Change Diagrams Ecolog