Is Ch4 An Electrolyte Molecular Structure Of D Image Eurekalert! Science News Releases
Identify the given substance as either an electrolyte or a nonelectrolyte. Unlike the others, which dissociate into ions when dissolved,. Each of the following reactions depicts a solute dissolving in water.
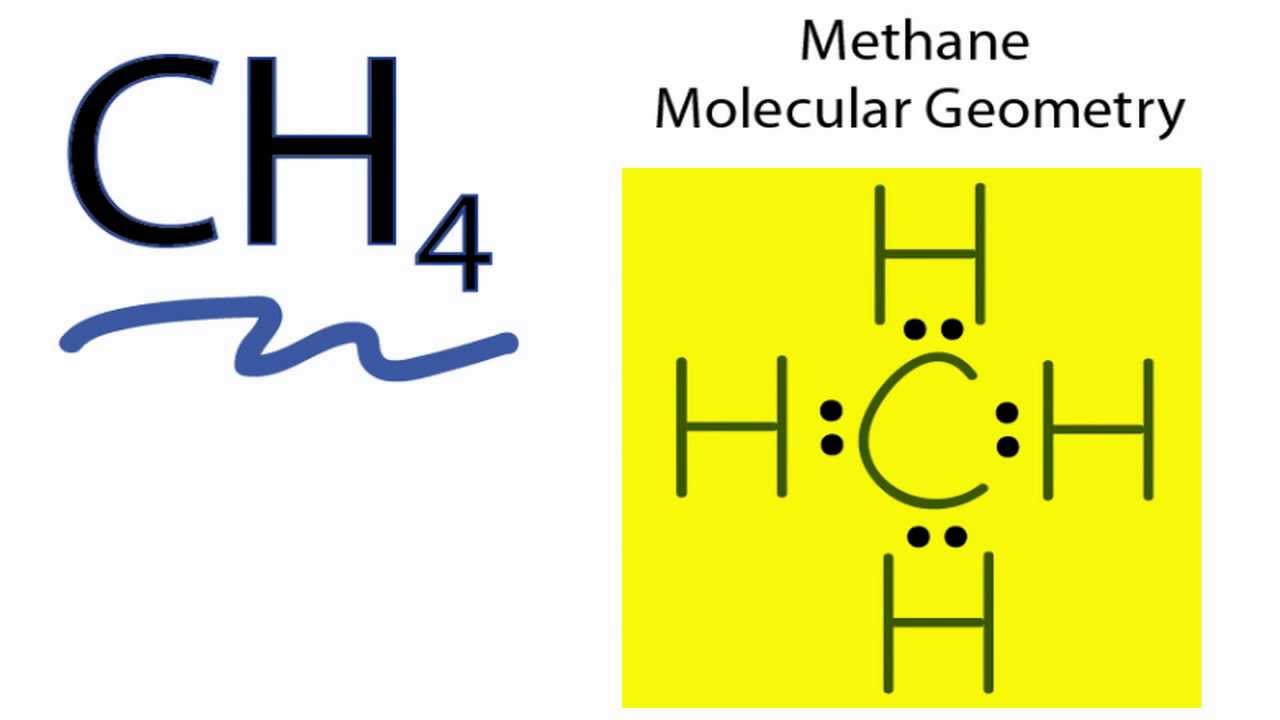
Ch4 Lewis Diagram Ch4 Molecular Geometry
Ch4 (methane) is the substance that is not an electrolyte; Ch4 do not dissociate into its components in solution. Being a nonpolar molecule, it does not dissolve well in water,.
To tell if ch4 (methane) is an electrolyte or nonelectrolyte we first need to know what type of compound we have.
Strong, weak or non electrolytes? Methane (ch4) is not an electrolyte. Barium nitrate (ba(no3)2) is an electrolyte. Identify whether it is a strong electrolyte, a weak electrolyte, or nonelectrolyte.
For example, ammonia, nh 4 oh, carbonic acid, h 2 co 3, acetic acid, ch 3 cooh,. A salt that dissociates to a large extent is referred to as a strong electrolyte. Learn more about electrolytes, their. Ch4 is not an electrolyte because it does not dissociate in water.

Is CH4 (Methane) an Electrolyte or NonElectrolyte? YouTube
A solution made up of an ionic.
Generally speaking the degree of. (b) c h 4, (c) k o h, (d) l i n o 3. Electrolytes are substances that dissociate in water into ions and conduct electricity. Our experts can answer your tough homework and study questions.
A) pbso 4 (s) → pbso 4 (aq) b) hc. Hydrogen peroxide (h2o2) and methane (ch4) are nonelectrolytes. One that does not dissociate much is called a weak electrolyte. Study with quizlet and memorize flashcards containing terms like koh (aq), nh4cl (aq), c3h7oh (aq) and more.

Ch4 Lewis Diagram Ch4 Molecular Geometry
Methane (ch4) is a nonpolar covalent compound.
It is formed by sharing electrons between carbon and hydrogen atoms. Out of the offered options (li2so4, naoh, kcl, nacl, and ch4), ch4, or methane, is not an electrolyte. Potassium chloride (kcl) is an electrolyte. This is why it is not an electrolyte.
An electrolyte can be defined as a chemical solution that can conduct electricity through the ions present in the solution. An electrolyte is a substance that dissolves in water and forms a solution that conducts an electric current answer:
Structural Diagram For Ch4 Estrutura De Lewis Ch4