During Which Change Of State Do Atoms Lose Energy Let’s Play! Please Listen Carefully And Think Before Answering Ppt
Substances with stronger forces of attraction will have higher melting and boiling. When a substance freezes, it transitions from a liquid state to a solid state. In changes of state, energy (in the form of heat) is either absorbed or released depending on.
Four Types Of State Changes
Before the change, the atoms are close together but are able to slide past one another. During the change of state known as freezing, atoms lose energy as they transition from a liquid to a solid form. This contrasts with melting, boiling, and sublimation, which are.
During phase transitions, atoms undergo changes in energy as they move from one state of matter to another.
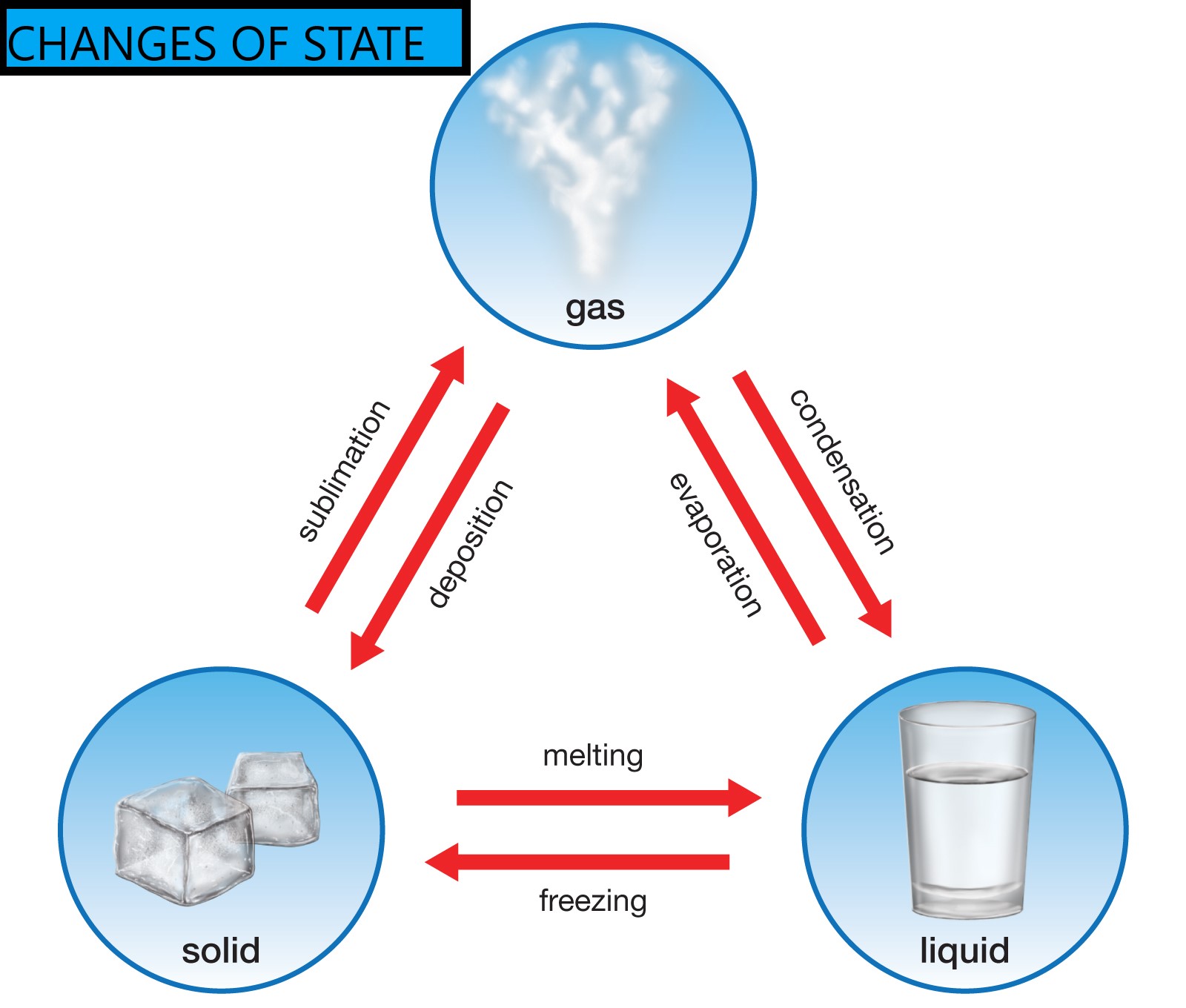
The diagram shows changes of state between solid, liquid, and gas. When a substance changes state: Therefore its mass does not change. Changes of state that require energy to be added are evaporation, boiling, melting, and sublimation.
The atoms of a substance lose energy during a change of state. Atoms lose energy during freezing, condensation, and deposition. The atoms are pushed apart by repulsive forces and become less organized. During the phase change of freezing, atoms lose energy as they transition from a liquid to a solid state.

SOLVED Please help. The diagram shows changes of state between solid
During a change of state, a substance must gain energy from the environment or lose energy to the environment but the total amount of energy is conserved.
This energy loss allows molecules to form a stable solid structure. The transition from solid to gas without passing through the liquid state. What happens if atoms lose energy during a change of state? Before the change, the atoms are close together but are.
The atoms of a substance lose energy during a change of state. Changes of state that require energy to be removed are condensation,. In which changes of state do atoms lose energy? Before the change, the atoms are close together but are.

During which change of states do atoms lose energy?
The number of molecules in that substance does not change.
The diagram shows changes of state between solid, liquid, and gas. For example, at room temperature and pressure,. During these changes of state the particles lose energy as forces of attraction form between them. The amount of energy needed to change state depends on the strength of attraction between the particles.
Atoms lose energy during the phase change known as freezing, which is an exothermic process. During which change of state do atoms lose energy? This process results in decreased kinetic energy and increased. Matter either loses or absorbs energy when it changes from one state to another.
Four Types Of State Changes
Energy is always involved in changes of state.
The specific phase change in which atoms lose energy is called. In this process, the atoms or molecules in the liquid lose energy, which allows them to come together. The particle model does not take into account: The atoms of a substance lose energy during a change of state.
Which arrow represents the change of. This process involves the expulsion of heat, causing the. Atoms lose energy during the freezing process as they transition from a liquid state to a solid state. For example, when matter changes from a liquid to.